Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Mizuta, Shunji; ;
JNC TN9400 2000-032, 38 Pages, 2000/03
lt is necessary for feasibility study of fast reactor to evaluate the oxidation of the austenitic stainless steels in the case of using for core material in carbon dioxide gas-cooled reactor. The properties for oxidation of austenitic stainless steels in carbon dioxide were surveyed in literatures and the data were selected after evaluation of factors for oxidation in carbon dioxide. The equation of oxidation in carbon dioxide for PE16, 20Cr/25Ni/Nb, 18Cr-8Ni and JNC Cladding materials were proposed. The equation for oxidation of austenitic stainless steels were expressed as upper limit for the equation according to parabolic law. The equation for JNC cladding materials (PNC316, PNC1520, 14Cr-25Ni) was proposed based the oxidation behavior of 18Cr-8Ni which is same oxidation region for weight gain in three-component system of Fe-Cr-Ni, in addition to evaluate of effect for silicon content. The oxidation equation of 20Cr/25Ni/Nb was applied to the high Ni alloy of JNC cladding material. The obtained equation is as follows, X = 4.4W 1000, W =
1000, W =  , kp =
, kp =  exp(-Q/(RT)), X: oxide thickness[
exp(-Q/(RT)), X: oxide thickness[ m], W : weight gain[g
m], W : weight gain[g cm
cm ], kp : parabolic rate constant[g
], kp : parabolic rate constant[g
 cm
cm
 s
s ], t :time[sec]
], t :time[sec]  : constant[g
: constant[g
 cm
cm
 S
S ], Q : activation energy[J・mol
], Q : activation energy[J・mol ], R : gas constant[8.314J
], R : gas constant[8.314J  K
K
 mol
mol ], T : temperature[K] (1) PE16 : kp = 1.090
], T : temperature[K] (1) PE16 : kp = 1.090 10
10 exp(-192,500/(RD)), (2) 20Cr/25Ni/Nb : kp = 1.651
exp(-192,500/(RD)), (2) 20Cr/25Ni/Nb : kp = 1.651 10
10 exp(-201,300/(RT)) High Ni alloy (JNC), (3)18Cr-8Ni : kp = 1.503
exp(-201,300/(RT)) High Ni alloy (JNC), (3)18Cr-8Ni : kp = 1.503 10
10 exp(-60,000/(RT)), (4) PNC316, PNC1520 : kp = 1.503
exp(-60,000/(RT)), (4) PNC316, PNC1520 : kp = 1.503 10
10 exp(-60,000/(RT))
exp(-60,000/(RT)) 0.62
0.62 14Cr-25Ni(JNC) The weight gain is (3)
14Cr-25Ni(JNC) The weight gain is (3)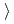 (4)
(4)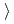 (2)
(2)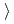 (1) in order.
(1) in order.